A lytic polysaccharide monooxygenase-like protein functions in fungal copper import and meningitis. - Nat. Chem. Biol. - X-MOL
Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components

F o r t e g n e l s e. over de kandidater, der er opstillet. til kommunalbestyrelsesvalget den 19. november 2013 i Gentofte Kommune - PDF Free Download

Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions – topic of research paper in Biological sciences. Download scholarly article PDF and read for free on CyberLeninka open
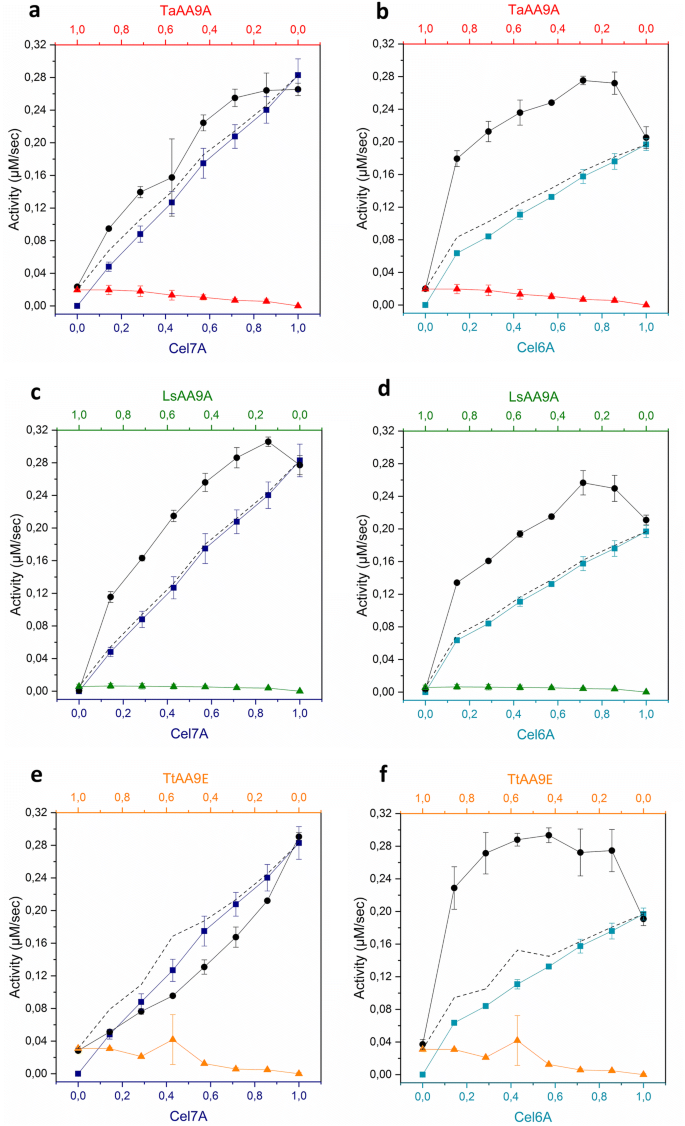
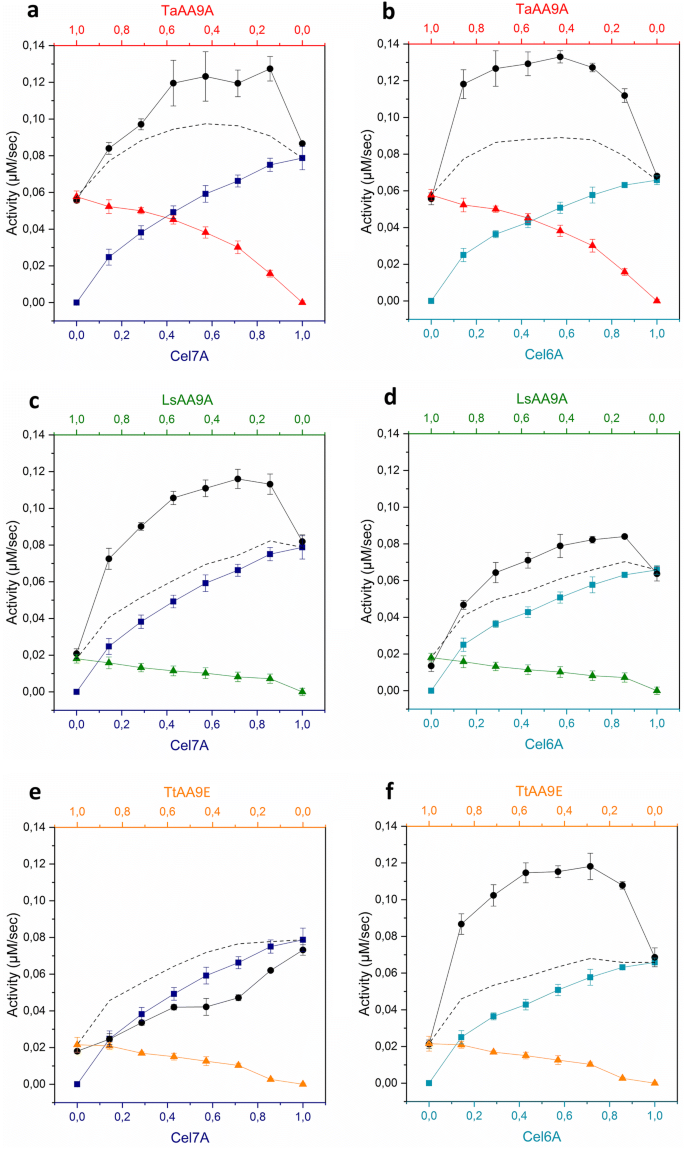
The synergy between LPMOs and cellulases in enzymatic saccharification of cellulose is both enzyme- and substrate-dependent | SpringerLink

A fungal family of lytic polysaccharide monooxygenase-like copper proteins. - Nat. Chem. Biol. - X-MOL
Ensiling and hydrothermal pretreatment of grass: consequences for enzymatic biomass conversion and total monosaccharide yields